Biodegradable PEG Dendrimers
Creative PEGWorks is now offering a series of PEG Dendrimers with biodegradable building blocks. Dendrimers are functionalized with amine, acid, azide etc.

Creative PEGWorks is now offering a series of PEG Dendrimers with biodegradable building blocks. Dendrimers are functionalized with amine, acid, azide etc.
Are you looking for a better way to improve your PEGylation yield? I bet you are. What Affects PEGylation Yield? Many parameters affect PEGylation process. The surface accessibility of the reactive site of amino acids is the most critical. On protein surfaces, there exist hydrophobic patches and concaves that expel water and thus hydrophilic PEGs. […]
Liposome Drug Products Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation Guidance for Industry It provides specific labeling requirements for liposome versus pegylated liposome. The nonproprietary name of a drug product approved under the Federal Food, Drug, and Cosmetic Act is its established name, which , in most instances , will be […]
A short polyethylene glycol (PEG) and its pegylation to small molecules could make a huge difference. Nektar announced positive topline results from an oral Human Abuse Potential (HAP) study of NKTR-181, which is a first-in-class opioid analgesic. What Is Nektar? NKTR-181 is a pegylated small molecule drug: a short PEG with six repeating ethylene glycol […]
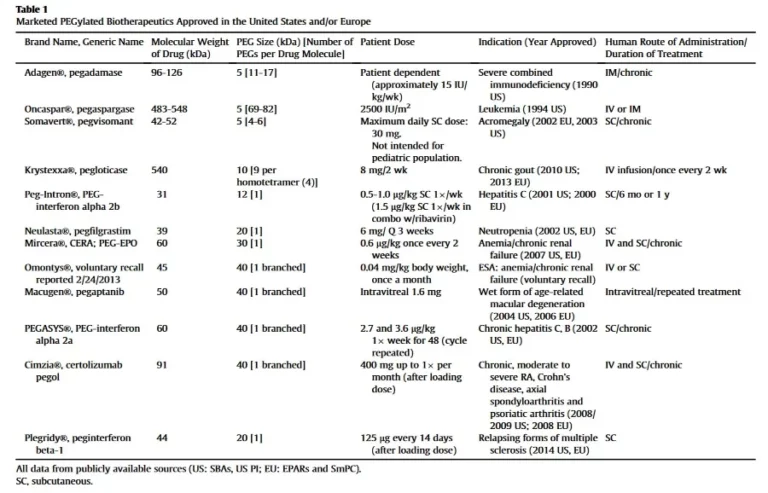
An excellent review article is published by scientists from Nektar Therapeutics, Bayer, Jansen, and Baxalta Innovations GmbH in the journal of Journal of Pharmaceutical Sciences. http://dx.doi.org/10.1016/j.xphs.2015.11.015 Journal of Pharmaceutical Sciences 105 (2016) 460-475 “PEGylation of Biopharmaceuticals: A Review of Chemistry andNonclinical Safety Information of Approved Drugs”. Since 1990, 12 PEGylated biopharmaceuticals have been introduced […]
Do you need a special PEG reagent synthesized? You’re in luck! At Creative PEGWorks, we handle custom synthesis services for polyethylene glycol (PEG) and other polymers. Common Custom Synthesis Requests Working with a wide variety of functional groups and a broad range of molecular weights, we frequently provide custom synthesis of: When you’re ready to […]
Benefits of PEGylation in Pharmaceuticals Wondering if you should use PEGylation for your products? There are a number of reasons to start using the PEG polymer, including the following benefits: If you’re looking for a company to handle your PEGylation needs, contact Creative PEG Works, a biotechnology company located in North Carolina. We specialize in […]
A recent article published in the leading scientific journal of Nature (Designer matrices for intestinal stem cell and organoid culture, Matthias P. Lutolf et al from the Ecole Polytechnique Fédérale de Lausanne (EPFL) in Switzerland, Nature, Published online 16 November 2016, doi:10.1038/nature20168) reported the first intestinal and colorectal cancer organoid culture in fully defined extracellular matrix […]
As a company that values your PEG products and is concerned about the quality of what you produce, it’s important that you use only the best PEG supplies. It can be difficult to find supplies and materials that are affordable but meet your quality standards. At Creative PEGWorks, we know the importance of quality supplies […]
The Best Laboratory Practices Certain aspects can get lost in the shuffle when performing day-to-day tasks, especially when you work in an environment that juggles various tasks like a laboratory. That is why having a set of practices is so important. Here are a few of the best practices you will find in the best […]
