Calculating the Contour Length and Hydrodynamic Radius of Polyethylene Glycol in Aqueous Solutions
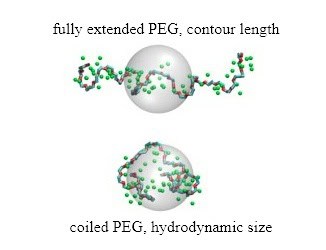
This post outlines the fundamental methods for calculating the contour length—the maximum extended chain dimension—and the hydrodynamic radius, which characterizes the effective size of the solvated coil. These calculations rely on established polymer physics and empirical relations from experimental data.