Creative PEGWorks developed a series of amphiphilic, surfactant-like PEG derivatives and they are now available…

Designer PEG Polyrotaxane Systems for CRISPR Gene Delivery
Recent research suggests PEGs can be used to optimize gene editing and gene delivery. Clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9 (CRISPR/Cas9) system employs a guide RNA (gRNA) which directs the Cas9 endonuclease to a specific site in the genome, and the Cas9 cleaves specific strands of DNA that are complementary to the CRISPR sequence, generating a double-stranded DNA break.
In vitro cell-based experiments are so promising; while in vivo delivery of CRISPR/Cas9 remains a significant challenge. Efficient delivery and gene editing with the CRISPR/Cas9 system requires co-delivery of the gRNA and the Cas9 endonuclease within the same cell to a particular part of the human body. The two components may be co-delivered as a plasmid encoding Cas9 and gRNA, Cas9 mRNA and gRNA, or as a ribonucleoprotein (RNP) in which the Cas9 protein is pre-assembled with the gRNA.
Systemic Delivery with Nanoparticles
Non-viral carriers, nanoparticles, represent a promising vehicle for co-delivery of the CRISPR/Cas9 system in either of the three formats. Nanoparticles are nanometers in size and can be designed for colloidal stability, tissue specificity (e.g., to skeletal muscle and muscle stem cells), biodegradability and biocompatibility.
Nanoparticles are largely non-immunogenic, a critical feature enabling them for repeat dosing. Nanoparticles composed of lipids, cationic polymers, mesoporous silica, gold, exosomes, metal-organic frameworks (MOF), and liposome, polymersome, polyethyleneimine (PEI), poly(methyl methacrylate) (PMMA), atelocollagen, and perfluorocarbon (PFPE) systems, etc are generally the choice for developing nano-CRISPR systems.
Currently, over 50 nanomedicines are FDA approved with many more in ongoing clinical trials primarily in oncology immunotherapy.
Exploring PEG-Based Nanocarriers
Systemic delivery of CRISPR/Cas9-mediated gene therapies remains a substantial obstacle particularly for neuromuscular disorders, such as Duchenne muscular dystrophy, because muscles comprise about 40% of the total body mass. Researchers at the University of California at Los Angeles (UCLA) developed biocompatible multi-arm PEG and linear PEG-based polyrotaxane nanocarriers, iteratively optimized for packaging large plasmid DNA for delivery to muscle cells.
Polyrotaxane Nanocarriers: the Plasmid DNA Delivery System
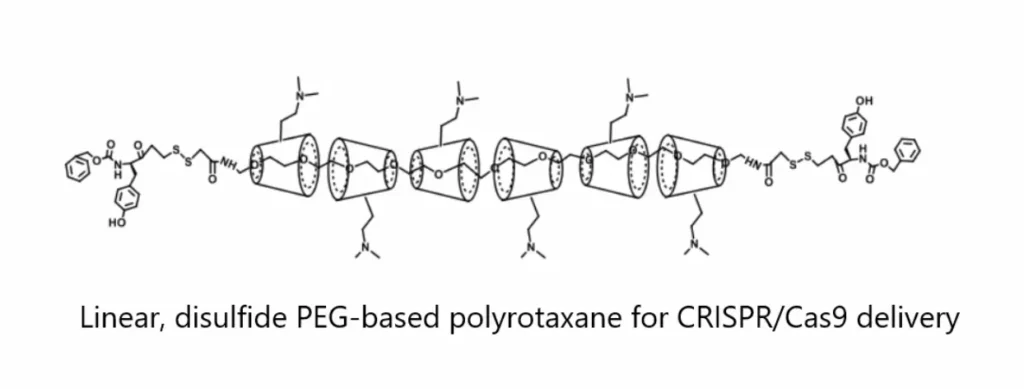
The polyrotaxane is optimized by the addition of a disulfide-responsive linker that enhances plasmid release. Furthermore, the conjugation of peptides leads to quicker uptake and improved transfection efficiency in humanized dystrophic muscle cells. Their work represents the first polyrotaxane platform that is optimized and designed for the delivery of large plasmid DNA, such as CRISPR/Cas9, to dystrophic muscle cells.
Polyrotaxane is synthesized by a mechanically interlocked molecule containing a polymer, such as a polyethylene glycol (PEG) backbone, with macrocycles, such as cyclodextrin rings, threaded onto the polymer and stabilized by bulky end groups. The addition of cationic charge on the macrocycles allows for effective complexation of nucleic acid mediated by electrostatic interactions.
PEG-Based Gene Delivery Research
UCLA custom-designed a 4-arm and 2-arm linear polyrotaxane nanocarrier that was engineered for improved circulation and pharmacokinetics (PK) following intravenous (IV) injection. Engineering polyrotaxane with a redox-sensitive disulfide linker improves plasmid release, which leads to improved gene delivery.
Their data also demonstrated proof-of-concept that the PEG-based polyrotaxane nanoparticles can deliver CRISPR/Cas9 to muscle cells and achieve a CRISPR-mediated deletion of DMD exons. 4-Arm PEG-Amine and Amine-PEG-Amine were used as the starting material to prepare polyrotaxanes. OPSS-PEG-OPSS was used to introduce pyridyldithiol forming the redox reactive disulfide bond as a reversible PEGylation strategy to better release the DNA payload and/or enable the polyrotaxane to dissociate once reached the target cells.
Conclusion: PEGs in Gene Delivery
The use of PEGs in systemic gene delivery is evolving. With many potential applications and ways to make current systems more efficient, now is the time to innovate. If you’re looking to start or continue your research with PEGs, explore our large variety of PEG products to better design PEG-based gene delivery nanocarriers! Call: 833-PEGWORK with any questions, place an order online or contact us for custom synthesis services!
Related Posts
- Surfactant-like PEG Derivatives for Drug Delivery
- PEGylated Hyaluronic Acid Nanoparticle Drug Delivery
A research group from the Republic of Korea developed poly(ethylene glycol)-conjugated hyaluronic acid nanoparticles (PEG-HANPs)…
- Surfactant-like PEG Derivatives for Drug Delivery
Creative PEGWorks developed a series of amphiphilic, surfactant-like PEG derivatives and they are now available…
