FDA has approved AstraZeneca’s Movantik for opioid-induced constipation in adults with chronic non-cancer pain. Movantik…

Is a PEGylated Protein Considered a Biobetter Biologic or Biosimilar Drug?
Legislation establishing an abbreviated approval pathway for biosimilar drugs was originally signed into law in March 2010 in the US. There have been many similar but confusing terminologies used to describe follow-on biologic drugs from the originator drug: biosimilar, biobetter, biogenerics, biosuperiors, second and third generation biologics.

What are Biosimilar Drugs?
Biosimilar drugs are generic versions of an innovator (or originator) biologic with the same amino acid sequence, but produced from different clones and/or manufacturing processes, leading to different glycosylation and other variations of low-level. It is currently not possible to produce exact copies of large proteins with significant post-translational modifications (PTM), particularly glycoproteins.
What are Biobetter Drugs?
Biobetter drugs are modified from an originator to generate functional changes that include increased half-life, reduced toxicity, reduced immunogenicity, and/or enhanced pharmacodynamics; have been engineered to have improved properties, e.g., optimized glycosylation profiles or an engineered Fc domain to increase the serum half-life of antibody therapy. Biobetter represents an opportunity for innovation with reduced risk already having a validated market, and the mechanism of action of the originator biologic is already known.
How Are Biobetter Drugs Made?
One of the main tools for the development of biobetters is PEGylation, a technique in which at least one chain of polyethylene glycol (PEG) is covalently attached to the structure of a protein. PEG is a biocompatible polymer, which presents minor immunogenicity, antigenicity or toxicity, and is soluble in water and many organic solvents.
These desirable traits render PEG one of the few easy-to-handle synthetic polymers for convenient and simple conjugation chemistry to a variety of biologic molecules including proteins, antibodies, enzymes, and nucleic acids.
A Brief History of PEGylation
PEGylation was first reported in 1977 for the modification of albumin and catalase. Depending on the number, molecular weight and location of the attached PEG chains, PEGylation can enhance the physicochemical properties of the protein, without compromising their secondary structure.
PEGylation provides a number of advantages for protein conjugates:
- (i) protection of antigenic epitopes;
- (ii) prevention of in vivo degradation by proteolytic enzymes;
- (iii) increased hydrodynamic size and volume that reduces renal filtration and increases in vivo half-life;
- (iv) increased water solubility and reduced protein aggregates.
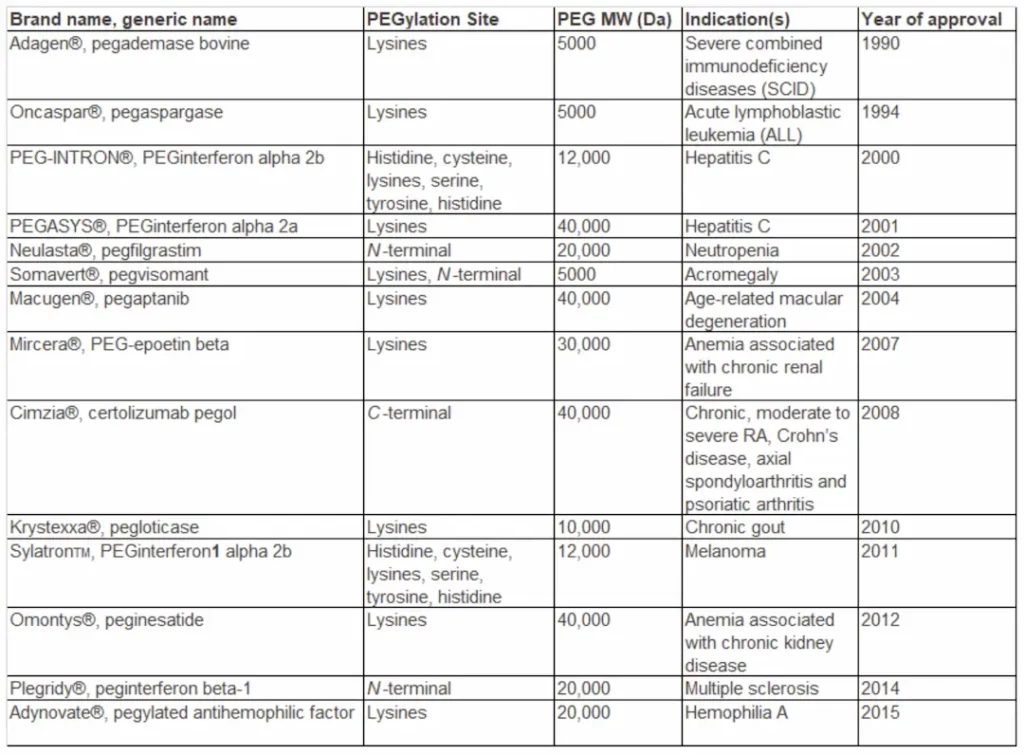
After the first report of protein PEGylation in the 1970s, many proteins and peptides have been covalently conjugated with PEG. To date, 14 biobetters (PEGylated proteins, peptides, antibody fragments, and oligonucleotides) have been approved by FDA and are currently on the market (Appendix), among which 12 are PEGylated proteins.
PEGylation was referenced in FDA documents such as FDA’s guidance that addresses the scientific and quality considerations for demonstrating biosimilarity to a reference protein product; and PEGylation was referenced as an example of post-translational modifications among phosphorylation, glycosylation, lipid attachment and others.
FDA on Biosimilar Drugs
According to the FDA, assessment of comparability with a reference product include molecular weight, higher order structure and post-translational modifications; the degree of heterogeneity; functional properties; impurity profiles; and degradation and stability profiles. Changes in post-translational modifications could substantially alter a protein’s activity. Therefore, a sponsor must demonstrate that across all of these characteristics there are no meaningful differences between the biosimilar and the reference product.
Does this open the door for a PEGylated protein to be considered as a biosimilar to an approved PEGylated protein drug or even a non-pegylated biologic drug? If a pegylated protein can be demonstrated biosimilar to another biologic drug, will FDA approve it through the Biosimilar approval pathway that was established by the Biologics Price Competition and Innovation Act (BPCI Act) of 2009?
Final Word: Biosimilar Drugs
The FDA’s consideration could open up the biologic drug research and development field and strengthen the use of PEGylation reagents. If you’re interested in starting your own biosimilar drug research, you can buy PEG products online from the leading PEGylation Reagent supplier.
Biosimilar Drug Reference Chart
Appendix – Commercially available PEGylated biologics on the market are listed in reverse chronology by FDA approval year:
Related Posts
- FDA Approves Constipation Drug PEGylated Naloxol
- PEGylated Hyaluronic Acid Nanoparticle Drug Delivery
A research group from the Republic of Korea developed poly(ethylene glycol)-conjugated hyaluronic acid nanoparticles (PEG-HANPs)…
- Surfactant-like PEG Derivatives for Drug Delivery
Creative PEGWorks developed a series of amphiphilic, surfactant-like PEG derivatives and they are now available…
