A recent review article discussed in details the current status and future prospect of protein…

Exciting Integration of PEGylation with Metal-Organic Frameworks: Advancing Biomedical Applications
Metal-Organic Frameworks
The Royal Swedish Academy of Sciences has decided to award Susumu Kitagawa, Richard Robson, and Omar M. Yaghi the Nobel Prize in Chemistry 2025, for the development of metal organic frameworks.
PEGylation
PEGylation refers to the covalent or non-covalent attachment of polyethylene glycol (PEG) chains to material surfaces, a modification that enhances biocompatibility, colloidal stability, and circulation half-life by reducing nonspecific protein adsorption and immune recognition.
PEGylated Metal-Organic Frameworks
Metal-organic frameworks (MOFs) constitute a class of porous, crystalline materials synthesized through the coordination of metal ions or clusters with organic ligands, forming one-, two-, or three-dimensional structures with exceptionally high surface areas and tunable porosity. These properties render MOFs suitable for applications in gas storage, catalysis, separation processes, and biomedical fields. PEGylation refers to the covalent or non-covalent attachment of polyethylene glycol (PEG) chains to material surfaces, a modification that enhances biocompatibility, colloidal stability, and circulation half-life by reducing nonspecific protein adsorption and immune recognition.
PEGylated MOFs integrate these attributes, yielding hybrid nanomaterials with improved performance in targeted therapeutic and diagnostic contexts. The PEG coating typically imparts hydrophilicity and steric hindrance, mitigating aggregation and facilitating aqueous dispersion, while preserving the inherent porosity of MOFs for cargo encapsulation.
Synthesis and PEGylation Strategies
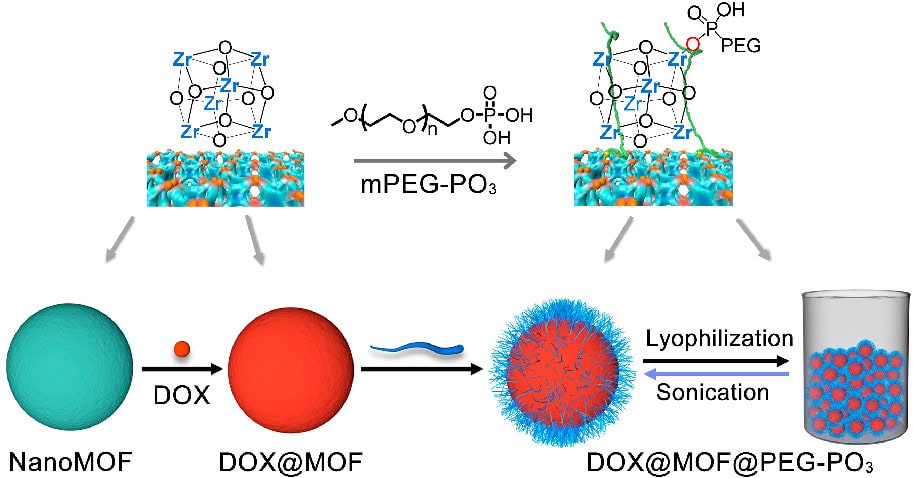
The synthesis of PEGylated MOFs typically employs post-synthetic modification to preserve the framework’s integrity. One approach utilizes coordination-based grafting, where PEG ligands bind to metal nodes, such as zirconium in UiO-66. For instance, selective surface PEGylation of UiO-66 nanoparticles is achieved through a “click modulation” method, incorporating azide or alkyne groups during synthesis followed by copper-catalyzed azide-alkyne cycloaddition to attach PEG chains. This technique controls particle size and surface chemistry while allowing cargo loading prior to modification.
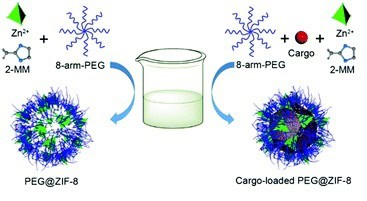
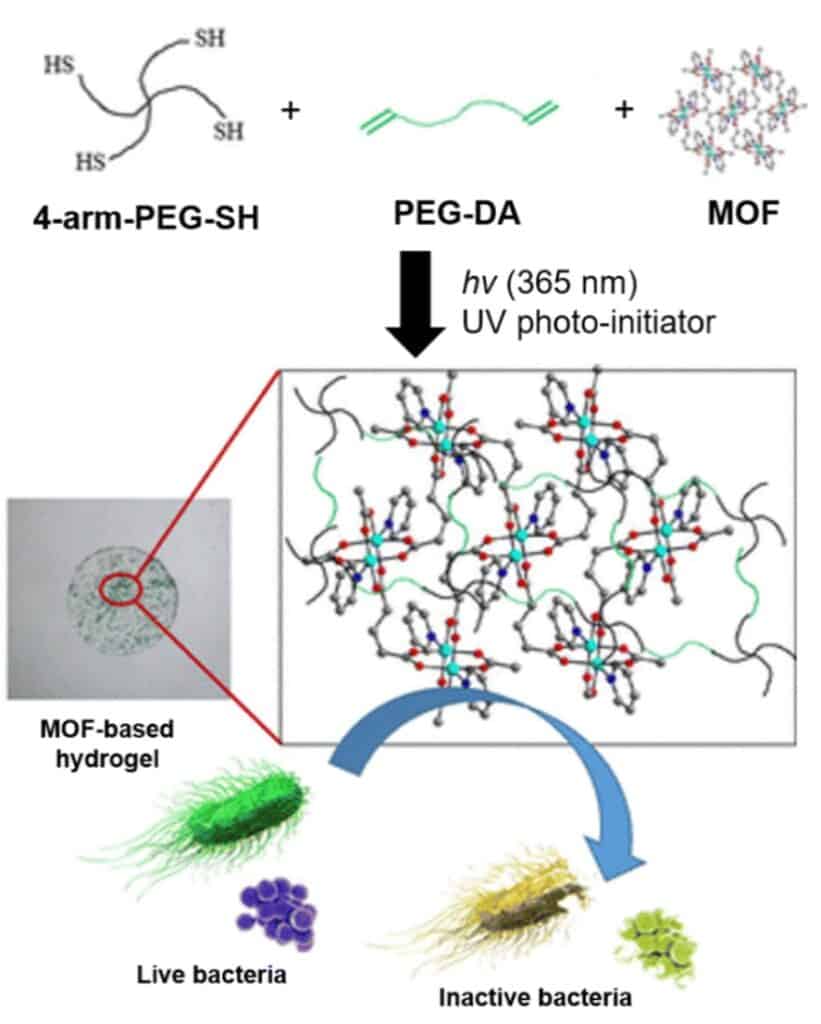
Alternative strategies include lipid bilayer integration, where UiO-66 is coated with a 1,2-dioleoyl-sn-glycero-3-phosphate lipid bilayer via sonication, incorporating PEG for enhanced stealth properties. Disulfide crosslinking with multi-arm PEG derivatives on ZIF-8 MOFs provides robust stabilization, often combined with hyaluronic acid for targeted delivery. Polymer coordination methods, such as attaching PEG to Zr-fum MOFs, leverage hydrogen bonding for shielding effects.
- Coordination-Based PEGylation: Methoxy PEG phosphate ligands coordinate directly with metal nodes (e.g., zirconium in UiO-66), promoting stable surface grafting. This approach yields nanoparticles with consistent hydrodynamic diameters, enhanced colloidal stability, and controlled drug release kinetics. Lyophilized forms of such PEGylated MOFs exhibit full redispersibility in water, addressing storage challenges for clinical translation.
- Polymerization Techniques: Nitroxide-mediated polymerization (NMP) enables the growth of PEG-styrene derivatives on amine-functionalized MOFs (e.g., UiO-66-NH₂), confirmed via attenuated total reflection infrared spectroscopy, time-of-flight secondary ion mass spectrometry, and powder X-ray diffraction. This method maintains crystallinity while minimizing nonspecific interactions.
- Encapsulation and Doping: For specialized applications, PEGylation follows the loading of therapeutic agents, such as disulfiram in copper-doped ZIF-8, where PEG modification occurs post-encapsulation to ensure tumor microenvironment (TME)-responsive release.
These methods ensure colloidal stability and compatibility with therapeutic agents, facilitating scalable production for clinical translation.
Benefits of PEGylation in MOF Systems
PEGylation confers several advantages to MOFs in biological environments. It prolongs circulation time by evading the mononuclear phagocytic system, reducing opsonization and immune recognition. For example, PEG-integrated lipid bilayers on UiO-66 extend blood half-life from 0.5 hours to 4 hours, improving biodistribution. Additionally, it minimizes protein adsorption and inhibits accelerated blood clearance, with multi-arm PEG structures showing minimal serum protein binding.
Enhanced stability in physiological conditions, such as phosphate-buffered saline, slows degradation and enables pH-responsive behaviors. PEG chains promote aqueous dispersion, reduce aggregation, and tune endocytosis pathways, favoring caveolae-mediated uptake to bypass lysosomal degradation. These properties collectively enhance the pharmacokinetics and therapeutic efficacy of MOF-based nanocarriers.
Applications in Drug Delivery and Cancer Therapy
PEGylated MOFs excel in targeted drug delivery, particularly for cancer theranostics. They enable high payload encapsulation of chemotherapeutics, imaging agents, and siRNA, with controlled release in the tumor microenvironment. A notable application involves UiO-66-PEG-F3, where covalent PEGylation and F3 peptide targeting facilitate doxorubicin loading and pH-responsive release, demonstrating preferential accumulation in breast cancer models via positron emission tomography imaging.
In environmental and separation contexts, PEGylated MOFs like UiO@GO@PEG selectively isolate glycoproteins through hydrophilic interactions. However, biomedical uses predominate, including biosensing, antiviral therapy, and immunotherapy enhancement.
- Drug Delivery and Cancer Theranostics: PEGylated nanoscale MOFs (nMOFs), such as Zr-based UiO-66 modified with fluorinated PEG, enable targeted delivery of chemotherapeutics and imaging agents. Positron emission tomography (PET) studies reveal preferential tumor accumulation in models like MDA-MB-231 xenografts, with faster hepatic clearance compared to noncovalent PEG variants. This enhances precision oncology by combining high payload capacity with pH-responsive degradation in acidic TME.
- Glycoprotein Isolation: PEGylated MOFs facilitate selective capture of immunoglobulin G from complex matrices, leveraging tunable surface chemistry for bioseparation.
- Anticancer Nanohybrids: PEG-DSF-Cu/ZIF-8 nanoparticles generate cytotoxic copper chelates and reactive oxygen species in situ, exhibiting ultrasensitive TME-triggered activation for precision treatment.
Environmental and Industrial Applications
- Heavy Metal Removal: PEG-functionalized Fe₃O₄@MIL-101(Cr) composites efficiently extract contaminants like lead and cadmium from herbal decoctions (e.g., Ligusticum chuanxiong Hort), with minimal impact on bioactive components (e.g., <0.2% solid content loss) and fingerprint similarity exceeding 99.9%.
- Desulfurization Membranes: Incorporation of MOF-505 into PEG matrices forms mixed-matrix membranes with superior sulfur removal efficiency, attributed to enhanced diffusivity and selectivity.
Emerging Uses
- Polymer Sorting: While not exclusively PEG-focused, MOF channels enable separation of PEG-terminated polymers by exploiting terminal group interactions.
- Wastewater Treatment: PEGylated MOFs contribute to pollutant adsorption (e.g., antibiotics, heavy metals), supporting circular economy initiatives.
| Application Area | Example MOF | PEG Modification Benefit | Key Outcome |
|---|---|---|---|
| Cancer Drug Delivery | UiO-66-PEG-F3 | Tumor targeting, rapid clearance | Enhanced PET imaging and payload release |
| Colloidal Stabilization | PCN-222@PEG-PO₃ | Redispersibility post-lyophilization | Stable storage for clinical use |
| Heavy Metal Extraction | Fe₃O₄@MIL-101(Cr)@PEG | Selective adsorption | Preserves herbal efficacy |
| Desulfurization | MOF-505/PEG Membrane | Improved permeability | Higher sulfur rejection |
Recent Examples and Studies
Recent advancements highlight innovative PEGylated MOFs for precision medicine. The PEGylated Elesclomol@Cu(II)-based MOF exhibits nanozyme activity, inducing cuproptosis in breast cancer cells by generating hydroxyl radicals and depleting glutathione. Combined with anti-PD-L1 antibodies, it suppresses tumor growth and boosts immunogenic responses.
Another example is the formulation of Zr-MOFs solidified via PEGylation, enabling drug-loaded composites with improved stability. These studies underscore the potential of PEGylated MOFs in overcoming biological barriers for effective cancer treatment.
Challenges and Future Directions
Despite progress, challenges include ensuring long-term in vivo stability, scalability, and minimizing immunogenicity. Future research may focus on stimuli-responsive PEGylation and hybrid systems integrating MOFs with other nanomaterials. Comprehensive reviews emphasize the need for reproducible synthesis to advance clinical applications.
Despite advancements, challenges persist, including long-term in vivo stability and scalability of synthesis. Ongoing research emphasizes dynamic PEGylation for stimuli-responsive behaviors and integration with other nanomaterials for multifunctional platforms. PEGylated MOFs hold substantial promise for translating porous frameworks into viable therapeutic agents, particularly in precision medicine and environmental remediation. For detailed methodologies, refer to primary literature on specific frameworks like UiO-66 or ZIF-8.
In conclusion, the use of PEG with metal-organic frameworks represents a promising synergy, enhancing their utility in targeted therapies and beyond. Continued investigation will likely yield transformative biomedical solutions.
Related Posts
- The Case for Protein PEGylation
- PEGylation Protocols and Literature References
Creative PEGWorks has collected hundreds of papers published in peer-reviewed journals citing the use of…
- PEGylation Reagents Available on Amazon!
Order PEG Products Online Creative PEGWorks set up a Merchant account on amazon.com to make…