FDA has approved AstraZeneca’s Movantik for opioid-induced constipation in adults with chronic non-cancer pain. Movantik…

FDA-Approved PEGylated Drugs
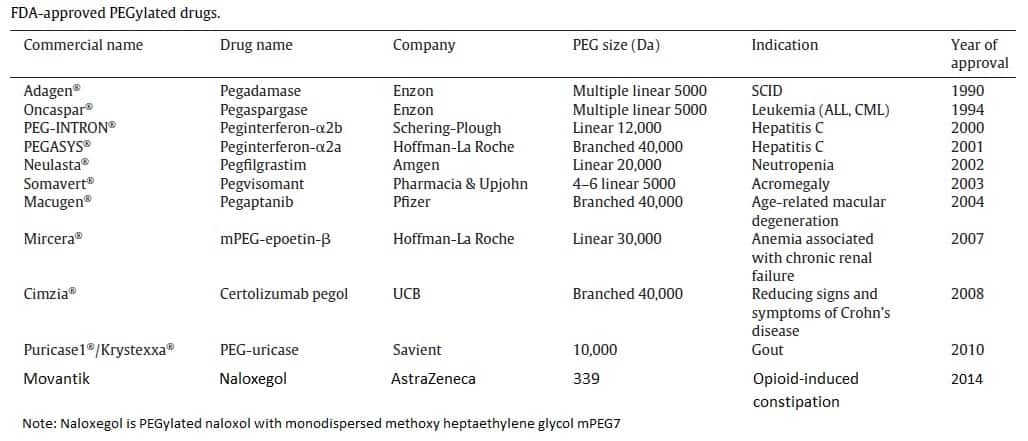
To date, only eleven PEGylated proteins, antibody fragments, oligonucleotides, and small molecules that have been approved by the FDA are on the market.
Available PEGylated Drugs
This includes PEGylated bovine adenosine deaminase: pegademase bovine (Adagen); and PEGylated l-asparaginase: pegaspargase (Oncaspar); PEGylated products of interferon-alpha (IFN-alpha): peginterferon-2b (PegIntron) and peginterferon-2a (Pegasys); PEGylated granulocyte colony stimulating factor (GCSF): pegfilgrastim (Neulasta); PEGylated growth hormone receptor antagonist: pegvisomant (Somavert); PEGylated a 28-nucleotide aptamer against vascular endothelial growth factor (VEGF); Pegaptanib sodium (Macugen); continuous erythropoietin receptor activator Mono-mPEG-epoetin- (Mircera); PEGylated Fab fragment of the humanized anti-tumor necrosis factor (TNF)-alpha monoclonal antibody certolizumab pegol (Cimzia); PEGylated recombinant porcine uricase (urate oxidase) (Puricase); and pegylated naloxol (naloxegol) (Movantik).
Current Drug Research on PEGylation with Small Molecule Agents, Progress in Polymer Science, 2013, 38, 421-444
Creative PEGWorks offers the most comprehensive collection of PEGylation reagents and PEG derivatives for the drug research community.
Related Posts
- FDA Approves Constipation Drug PEGylated Naloxol
- PEGylated Hyaluronic Acid Nanoparticle Drug Delivery
A research group from the Republic of Korea developed poly(ethylene glycol)-conjugated hyaluronic acid nanoparticles (PEG-HANPs)…
- Is a PEGylated Protein Considered a Biobetter Biologic or Biosimilar Drug?
The FDA’s consideration of a biosimilar drug could open up biologic drug research. Learn how…