A research group from the Republic of Korea developed poly(ethylene glycol)-conjugated hyaluronic acid nanoparticles (PEG-HANPs)…

FDA Approves Constipation Drug PEGylated Naloxol
FDA has approved AstraZeneca’s Movantik for opioid-induced constipation in adults with chronic non-cancer pain. Movantik (naloxegol), an oral once-a-day treatment originally developed by Nektar Therapeutics, can decrease the constipating effects of opioids. The drug’s safety and effectiveness were established in two clinical trials.
What Is PEGylated Naloxegol?
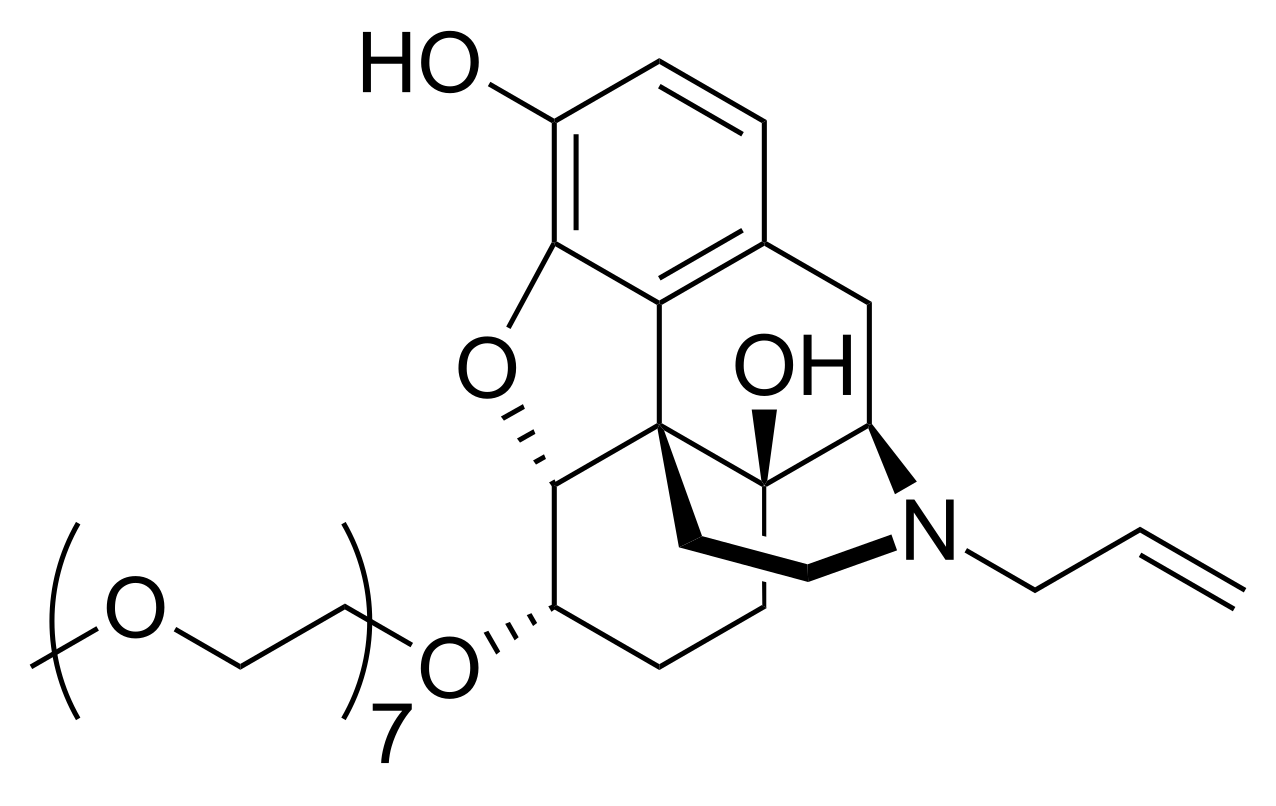
Naloxegol is PEGylated naloxol, which is a peripherally-selective opioid antagonist. It is a PEGylated small molecule drug with a circulation half-life of 6-11 hours and it is primarily excreted through the feces and urine. Its structure contains a methoxy capped heptaethylene glycol (mPEG7) conjugated to naloxol through an ether linkage.
- Molecular formula: C33H51NO11
- Molecular weight: 637.758
Looking for a PEG Supplier?
Creative PEGWorks supplies a large collection of monodisperse polyethylene glycol derivatives and PEG reagents. Our PEG compounds have all common organic reactive functional groups that can be directly conjugated to small molecule drugs, proteins, and other biologic drugs for the development of better medicine.
Related Posts
- PEGylated Hyaluronic Acid Nanoparticle Drug Delivery
- Surfactant-like PEG Derivatives for Drug Delivery
Creative PEGWorks developed a series of amphiphilic, surfactant-like PEG derivatives and they are now available…
- High-Density Lipoprotein Mimetic Drug Delivery Systems for Cancer, Cardiovascular Diseases
Nanolipoprotein particles (NLPs), which are mimetics of naturally occurring high-density lipoproteins (HDLs) present in the…
