Description
PEGylated small molecule drugs refer to therapeutic agents with molecular weights typically below 1,000 Da that have been covalently conjugated with polyethylene glycol (PEG) chains to optimize their pharmaceutical properties. PEG, a biocompatible, hydrophilic polymer composed of repeating ethylene oxide units, is attached via stable linkages such as amide, ester, or ether bonds to functional groups on the small molecule (e.g., amines, carboxylates, or hydroxyls). This modification transforms the parent compound into a prodrug with enhanced solubility, stability, and pharmacokinetic performance.
Mechanism of Pharmacokinetic Enhancement, Upon administration, the PEG moiety extends plasma half-life from minutes (for unmodified small molecules) to hours or days by minimizing rapid excretion and metabolic breakdown. This results in sustained therapeutic levels, reduced dosing frequency, and improved bioavailability. In some cases, PEGylation facilitates passive tumor targeting via the enhanced permeability and retention (EPR) effect in oncology applications.
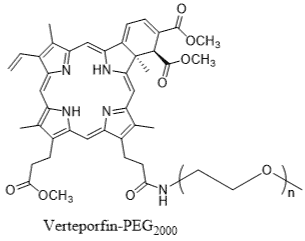
Representative Examples: PEG-Naloxol (Narcan® variant): PEGylated opioid antagonist for overdose reversal, improving duration of action. PEG-Doxorubicin Conjugates: Investigational anthracycline derivatives for cancer, reducing cardiotoxicity while maintaining efficacy. PEG-Verteporfin: a photosensitizer with PEG chains for enhanced circulation in photodynamic therapy.
PEGylation represents a mature platform technology that has revolutionized small molecule therapeutics by addressing key limitations in solubility, pharmacokinetics, and patient compliance. Ongoing advancements focus on releasable linkers and stimuli-responsive PEGs for controlled drug release.