
4-Arm PEG-SC (SC: Succinimidyl Carbonate)
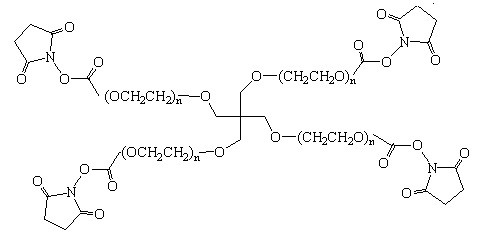
Properties
Molecular weight: 4-Arm PEG MW refers to the MW of the entire PEG molecule. The MW of each arm is 1/4 of the MW indicated in the product name. MW of PEG was measured by MALDI-MS or GPC. PDI (polydispersity index) of our 4-Arm PEG is 1.02-1.05 with very narrow MW distribution. The number of repeating ethylene oxide units (CH2CH2O) or the degree of polymerization is calculated dividing the PEG MW by 44 (44 is the molecular mass of one repeating unit).
Solubility: Soluble in water and aqueous buffer, chloroform, methylene chloride, DMF, DMSO, and less soluble in alcohol, toluene. Not soluble in ether.
Density: PEG density is approximately 1.125 g/mL.
Physical form: PEG products generally appear as white or off-white powder, and for very low MW 4-Arm PEG such as MW 2k, it may appear as wax-like, semi-solid material due to the low MW and the type of functional groups.
Storage condition: PEG product shall be stored in the original form as received in a freezer at -20C or lower for long term storage. Stock solution of PEG reagents that do not contain oxygen or moisture sensitive functional groups may be temporarily stored in a refrigerator or ambient temperature for multiple days. Stock solution should avoid repeated freeze-and-thaw cycles. See Documents section for detailed storage and handling conditions.
| PEG NHS esters | Structural characteristics | Amine reactivity | Stability |
|---|---|---|---|
| Type A: SCM | Methylene (CH2) linkage between PEG and NHS ester | Highly reactive | Hydrolysis half-life: less than five minutes. It often requires the use of a large excess of PEG reagents. |
| Type B: SG | C4 aliphatic ester linkage between PEG and NHS ester | Very reactive | Hydrolysis half-life: around 20 minutes. |
| Type C: SS | C3 aliphatic ester linkage between PEG and NHS ester | Very reactive | Hydrolysis half-life: around 10 minutes. |
| Type D: GAS | C4 aliphatic amide linkage between PEG and NHS ester | Very reactive | Hydrolysis half-life: around 20 minutes. |
| Type E: SAS | C3 aliphatic amide linkage between PEG and NHS ester | Very reactive | Hydrolysis half-life: around 10 minutes. |
Get In Touch
If you have any questions, please submit an online inquiry.
"*" indicates required fields
