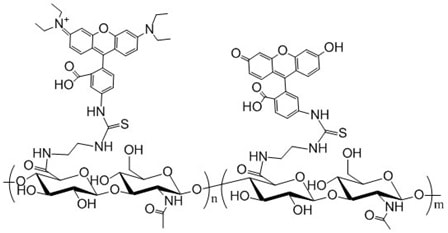
Description
Hyaluronic Acid is labeled with both rhodamine (Ex/Em wavelength 552/575 nm) and fluorescein (Ex/Em wavelength 494/518 nm). The rhodamine used in all of polysaccharide products is 5/6-carboxy-tetramethyl- rhodamine, (5/6-TAMRA). Purity: >95% lyophilized powder. Degree of substitution: 1 mol % substitution for each dye and at least one rhodamine and one fluorescein molecule per polymer.
Properties
Name and Source: Hyaluronic acid (HA, Hyaluronan, Hyaluronate) is an anionic, non-sulfated glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues. CAS# 9004-61-9. Our HA products are produced via microbial fermentation. See Documents section for details.
Appearance: Lyophilized powder. The color depends on the color of the dye and the dye labeling ratio.
Solubility: Soluble in water and the solubility data varies with MW. Higher MW results in lowered solubility. Typically solubility ranges 5-50 mg/mL, that is, 0.5%-5%. Buffered solution at pH >6 enhances solubility due to deprotonation of carboxylic acid groups and formation of carboxylate salts. HA derivatives with hydrophobic groups will lower the solubility in water, and adding alcohol or DMSO to an alcohol-water or DMSO-water mixture can dramatically improve the solubility up to 200 mg/mL (20%).
Degree of Substitution (DoS): The standard DoS is 1% by default. Product variants with special DoS other than 1% is indicated in the product name and catalog number. For example, HA-635-2% means that the DoS is 2% for this particular product.
The substitution or labeling ratio is the percentage of HA disaccharide monomers that are functionalized with the functional groups. You may calculate the number of functional groups in each HA polymer chain by 1) dividing the MW of HA by the MW of one disaccharide monomer to derive the number of disaccharide monomers in HA; 2) then multiple the number of disaccharide monomers by the labeling ratio or DoS to get the number of functional groups in each HA polymer. See Documents section for details.
References
1. Enhanced articular cartilage by human mesenchymal stem cells in enzymatically mediated transiently RGDS-functionalized collagen-mimetic hydrogels, Acta Biomaterialia, 2017, 51, 75-88, Text.
2. Targeted delivery of hyaluronic acid to the ocular surface by a polymer-peptide conjugate system for dry eye disease, Acta Biomaterialia, Volume 55, June 2017, Pages 163-171, Text.
3. Harnessing the Versatility of Bacterial Collagen to Improve the Chondrogenic Potential of Porous Collagen Scaffolds, Adv Healthc Mater. 2016;5(13):1656-66, Text.
4. Topical delivery of hyaluronic acid into skin using SPACE-peptide carriers, Journal of Controlled Release, 2014, 173(10), pp 67–74, Text.
5. A hyaluronic acid-binding contact lens with enhanced water retention, Contact Lens and Anterior Eye, 2014, 38(2), pp 79–84, Text.
6. Influence of high hydrostatic pressure on solid supported DPPC bilayers with hyaluronan in the presence of Ca2+ ions, Soft Matter, 2019,15, 7295-7304, DOI: 10.1039/C9SM01066A
7. Temporally degradable collagen–mimetic hydrogels tuned to chondrogenesis of human mesenchymal stem cells, Biomaterials, 99, 2016, 56-71, Text.
8. Hyaluronan modulation impacts Staphylococcus aureus biofilm infection. Infection and Immunity (2016): IAI-01418. Text.
9. Synergistic Antitumor Activity of Camptothecin-Doxorubicin Combinations and their Conjugates with Hyaluronic Acid. J Control Release. 2015 Jul 28;210:198-207.Text.
Click here to view an expanded list of hundreds of publications citing Creative PEGWorks products.