Description
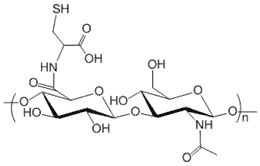
Hyaluronic Acid is functionalized with thiol groups. Purity: >95% powder. Standard Degree of substitution (DoS): 5 mol % substitution unless specifically noted in the catalog number. For example HA-371-30% means that the DoS of thiol in this hyaluronate product is 30%. HA-Thiol with lower or higher thiol, SH, sulfhydryl or mercapto substitution ratio (e.g. 1% and 10%) may be offered through custom synthesis service. Synonyms: HA, hyaluronate, hyaluronan.
Properties
Name and Source: Hyaluronic acid (HA, Hyaluronan, Hyaluronate) is an anionic, non-sulfated glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues. CAS# 9004-61-9. Our HA products are produced via microbial fermentation. See Documents section for details.
Appearance: White, lyophilized powder, except dye-labeled HA reagents.
Solubility: Soluble in water and the solubility data varies with MW. Higher MW results in lowered solubility. Typically solubility ranges 5-50 mg/mL, that is, 0.5%-5%. Buffered solution at pH >6 enhances solubility due to deprotonation of carboxylic acid groups and formation of carboxylate salts. HA derivatives with hydrophobic groups will lower the solubility in water, and adding alcohol or DMSO to an alcohol-water or DMSO-water mixture can dramatically improve the solubility up to 200 mg/mL (20%).
Degree of Substitution (DoS): The standard DoS is 5% by default. Product variants with special DoS other than 5% is indicated in the product name and catalog number. For example, HA-301-30% means that the DoS is 30% for this particular product.
The substitution or labeling ratio is the percentage of HA disaccharide monomers that are functionalized with the functional groups. You may calculate the number of functional groups in each HA polymer chain by 1) dividing the MW of HA by the MW of one disaccharide monomer to derive the number of disaccharide monomers in HA; 2) then multiple the number of disaccharide monomers by the labeling ratio or DoS to get the number of functional groups in each HA polymer. See Documents section for details.
References
1. PEGylated crosslinked hyaluronic acid nanoparticles designed through a microfluidic platform for nanomedicine, Nanomedicine, 2017, 12(18), Text.
2. Hyaluronic acid based scaffolds for tissue engineering—A review, Carbohydrate Polymers 2013, 92(2):1262– 1279; Text.
3. Single-molecule mechanical measurements of the hyaluronan-aggrecan bottle brush, Biomolecules, 2019, Text.
4. A Microfluidic Platform to design Multimodal PEG – crosslinked Hyaluronic Acid Nanoparticles (PEG-cHANPs) for diagnostic applications, Scientific Reports, volume 10, Article number: 6028 (2020), Text.
5. A role for mast cells in geographic atrophy, The FASEB Journal. 2020;00:1–15, Text.
6. Microbubbles Cloaked with Hydrogels as Activatable Ultrasound Contrast Agents, ACS Appl. Mater. Interfaces 2020, Text.
7. Unsaturated and thiolated derivatives of polysaccharides as functional matrixes for tissue engineering and pharmacology: A review, Carbohydrate Polymers 259 (2021) 117735, Text.
8. HA-Thiol/PEG-Methacrylate: In situ forming biomaterials as muscle void fillers for the provisional treatment of volumetric muscle loss injuries, Materials Today Bio, Volume 22, October 2023, 100781, Text.
9. Robust Synthesis of Targeting Glyco-Nanoparticles for Surface Enhanced Resonance Raman Based Image-Guided Tumor Surgery, Small Sci. 2024, 2300154, Link, Text.
10. Robust Synthesis of Targeting Glyco-Nanoparticles for Surface Enhanced Resonance Raman Based Image-Guided Tumor Surgery, Small Sci. 2024, 2300154, Text.
Click here to view an expanded list of hundreds of publications citing Creative PEGWorks products.