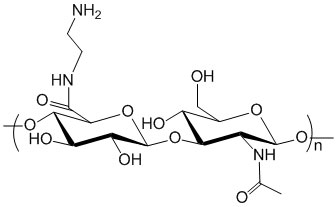
Description
Hyaluronic Acid is functionalized or derivatized with amine groups. Amine groups are conjugated to HA through an amide bond. Purity: >95% powder. Degree of substitution (DoS): 5 mol %; unless specifically noted in the catalog number. For example, HA-323-50% means that the DoS for amine is 50%. HA-Amine derivatives with lower or higher amine NH2 substitution ratio (e.g. 1% and 10%) may be offered through custom synthesis service.
Properties
Name and Source: Hyaluronic acid (HA, Hyaluronan, Hyaluronate) is an anionic, non-sulfated glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues. CAS# 9004-61-9. Our HA products are produced via microbial fermentation. See Documents section for details.
Appearance: White, lyophilized powder, except dye-labeled HA reagents.
Solubility: Soluble in water and the solubility data varies with MW. Higher MW results in lowered solubility. Typically solubility ranges 5-50 mg/mL, that is, 0.5%-5%. Buffered solution at pH >6 enhances solubility due to deprotonation of carboxylic acid groups and formation of carboxylate salts. HA derivatives with hydrophobic groups will lower the solubility in water, and adding alcohol or DMSO to an alcohol-water or DMSO-water mixture can dramatically improve the solubility up to 200 mg/mL (20%).
Degree of Substitution (DoS): The standard DoS is 5% by default. Product variants with special DoS other than 5% is indicated in the product name and catalog number. For example, HA-301-30% means that the DoS is 30% for this particular product.
The substitution or labeling ratio is the percentage of HA disaccharide monomers that are functionalized with the functional groups. You may calculate the number of functional groups in each HA polymer chain by 1) dividing the MW of HA by the MW of one disaccharide monomer to derive the number of disaccharide monomers in HA; 2) then multiple the number of disaccharide monomers by the labeling ratio or DoS to get the number of functional groups in each HA polymer. See Documents section for details.
References
1. Chemical modification of hyaluronic acid by carbodiimides, Bioconjugate chemistry, 1991, 2(4):232-41.
2. Hyaluronic acid based scaffolds for tissue engineering—A review, Carbohydrate Polymers 2013, 92(2):1262– 1279
3. Induction of Cancer Cell Death by Hyaluronic Acid-Mediated Uptake of Cytochrome C. J Nanomed Nanotechnol 6.316 (2015): 2. Text.
4. Bio-orthogonally crosslinked hyaluronate-collagen hydrogel for suture-free corneal defect repair. Biomaterials, 2020, 120176. DOI: 10.1016/j.biomaterials.2020.120176
Click here to view an expanded list of thousands of publications citing Creative PEGWorks products.